One of the reasons that asteroid mining is such a popular idea among industrialists is that they holds large quantities of heavy elements. NASA’s Psyche spacecraft just launched this week, headed for an asteroid that holds ten to thirty quintillion U.S dollars worth of rare-earth elements and other heavy metals. During our planet’s formation, the heavier of these elements tended to sink deep into the Earth, making them hard to find. But even in small asteroids, these heavy elements might be much more common and accessible. Since we rely on these rare-earth elements for our modern society, metal-rich asteroids such as Psyche are worth checking out.
But there are some who think a few asteroids might hold an even greater treasure: ultra-heavy elements. Elements well beyond Uranium on the periodic table. If they existed, these elements would be so dense they would have quickly sunk to Earth’s core in its youth, which is why they have never been found on Earth. It would be a tremendous discovery if true, but it could also be nothing more than the unobtanium of science fiction.
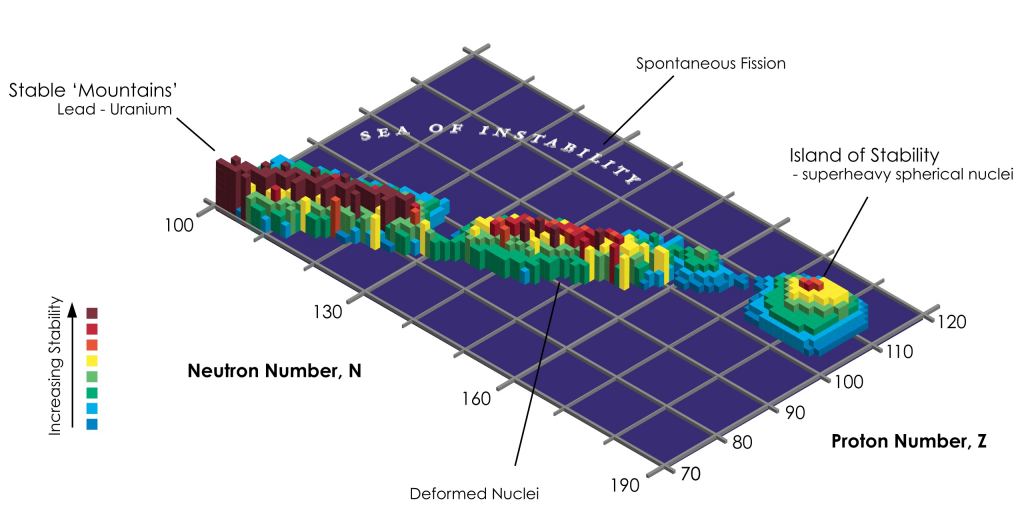
The idea of ultra-heavy elements comes from theoretical models of large atomic nuclei. Elements beyond iron (atomic number z=26) are not formed by fusion in stellar cores but by cataclysmic events such as supernovae and the collisions of neutron stars. That includes all the silver, gold, tin, and lead. Everything up to uranium, with an atomic number of 92. Elements beyond uranium might occur naturally, but they have never been found in nature.
Part of the reason for this is that for elements beyond lead, only thorium (z=90) has a stable isotope. Uranium has a half-life of a few billion years, plutonium (z=94) about 80 million years, and americium (z=95) just 7,000 years. After that the elements have half-lives on the order of days at best, and often just seconds or milliseconds. Very large nuclei just aren’t radioactively stable, so even if they occur naturally they don’t hang out in asteroids for billions of years.
But theoretical calculations of large nuclei suggest that well beyond uranium, around z=114, there could be an island of stable elements. These would have a “magic number” of protons and neutrons to fill nuclear shells, making them unusually stable. The island of stability isn’t a controversial idea, but most nuclear chemists figure that residents of this island will have half-lives no longer than a century. But some argue they could have half-lives similar to uranium, on the order of a billion years. If that’s the case, then elements such as flerovium might be lurking in the asteroids.
Interestingly, there is a bit of evidence for superheavy elements in meteorite fragments. A 2019 study looked at cosmic ray trails in fragments of meteoric olivine and found evidence of three trails suggesting they were made by superheavy elements with a half-life of a few decades. Other studies also suggest evidence of the island of stability, but nothing with billion-year lifetimes.
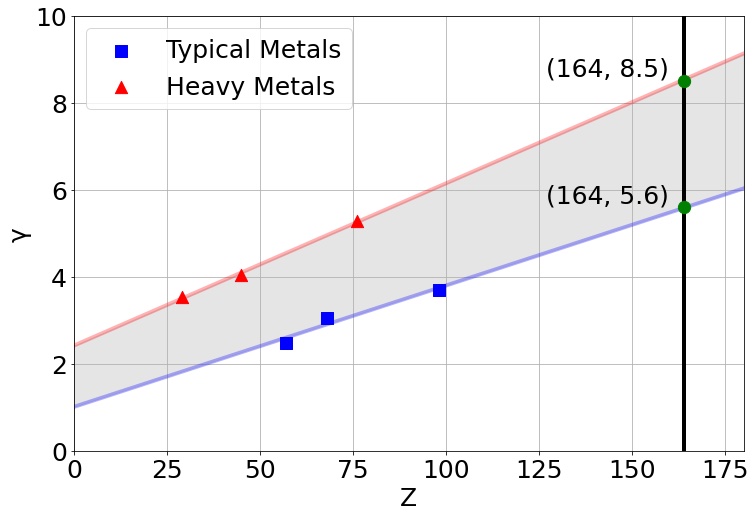
Which brings us to a new study making sensational headlines lately. The authors focus most of the paper on how superheavy and ultradense elements in the island of stability might form naturally without the need for dark matter. That by itself is interesting, but the authors also point to an asteroid known as 33 Polyhymnia as a likely source of such elements, which is a hugely wild claim.
Polyhymnia has not been widely studied, but a 2012 paper calculated its mass to be 6 x 1018 kg. Given that the asteroid is only 50 kilometers across, the estimated density would be 75 g/cm3, which is so wildly out of line that even the author of the paper considers it unreliable. The result hasn’t been confirmed by later observations. However, this new study notes that the result could be correct because it contains superheavy elements.
To say that argument is weak tea is putting it lightly. While the island of stability may be real, and there may be naturally occurring elements beyond uranium, there is no evidence that they are found in asteroids.
Reference: Alexandrov, Andrey, et al. “Natural superheavy nuclei in astrophysical data.” arXiv preprint arXiv:1908.02931 (2019).
Reference: LaForge, Evan, et al. “Superheavy Elements and Ultradense Matter.” The European Physical Journal Plus 138 (2023): 812.
Reference: Carry, Benoit. “Density of asteroids.” Planetary and Space Science 73.1 (2012): 98-118.
The post Can We Find the Heaviest Elements in Asteroids? appeared first on Universe Today.
No comments:
Post a Comment